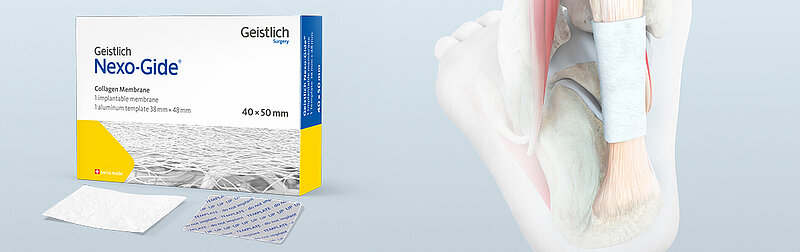
The Geistlich Nexo-Gide® Dual Surface Membrane is an absorbable, highly purified and bi-layered collagen membrane for the management and protection of tendon injuries. It serves as a protective cover to support the body's own healing process. The product is manufactured using a patented process, which results in structurally robust product properties. This membrane performs particularly well in constellations where there is no significant loss of tendon tissue.
Every second musculoskeletal injury is due to tendon and ligament injuries
"Tendon injuries are still a major clinical challenge," says Carrie Hartill, Business Unit Director at Geistlich Surgery. The increase in sports participation is a positive message. The downside of the phenomenon, especially in the older population, is the high number of tendon and ligament injuries. They account for nearly half of the 32 million musculoskeletal injuries in the U.S. each year. Clinicians strive for a higher likelihood of a positive surgical outcome, and to do so, they need a versatile, flexible option with clearly carved-out features. This is where Geistlich Nexo-Gide ® comes into its own and delivers its full performance. Geistlich Pharma is thus further expanding its range in regenerative medicine. The aim remains unchanged: patients gain an improved quality of life, while clinicians benefit from high application quality.